What is permanent hardness in water and how to remove it?
Water hardness refers to the content of dissolved calcium and magnesium ions in water. It can be divided into two types: temporary hardness and permanent hardness. Temporary hardness is caused by calcium carbonate and magnesium carbonate, which can be removed by heating water; permanent hardness is caused by calcium sulfate and magnesium sulfate, which cannot be removed by heating. Here's more about permanent hardness in water and how to remove it.
1. Causes of permanent hardness of water
The permanent hardness of water mainly comes from the calcium sulfate and magnesium sulfate in the water. When these substances are dissolved in water, they do not precipitate upon heating and therefore cannot be removed by simple heating. Permanent hardness will bring inconvenience to life and industrial production, so corresponding measures need to be taken to remove it.
2. Method of removal of permanent hardness of water
The main methods for removal of permanent hardness of water include ion exchange and adding complexing agents.
Ion exchange uses ion exchange resin to absorb calcium and magnesium ions in water and simultaneously releases an equal amount of sodium or hydrogen ions, thereby reducing the hardness of water. This is a common method of removing water hardness and is widely used in industrial and domestic water supplies.
Adding a complexing agent is to add a certain amount of complexing agents, such as phosphates, carbonates, etc., to the water to form insoluble compounds with calcium and magnesium ions in the water and precipitate, thereby reducing the hardness of the water. This method is simple to operate and low cost, but the dosage of additives needs to be controlled to avoid secondary pollution of water quality.
What methods are effective in removing permanent hardness of water?
The permanent hardness of water brings many inconveniences to life and industrial production, so effective methods need to be taken to remove it. Here are some effective ways to remove permanent hardness from water.
1. Ion exchange method
Ion exchange method is currently a widely used method to remove permanent hardness from water. The ion exchange resin absorbs calcium and magnesium ions in the water and releases an equal amount of sodium or hydrogen ions at the same time, thereby reducing the hardness of the water. This method is simple to operate and has obvious effects, and is widely used in industrial and household water use.
2. Adding complexing agent method
Adding complexing agents is another commonly used method to remove permanent hardness of water. By adding a certain amount of complexing agent to the water, it forms an insoluble compound with the calcium and magnesium ions in the water and precipitates, thereby reducing the hardness of the water. This method is simple to operate and low cost, but the dosage of additives needs to be controlled to avoid secondary pollution of water quality.
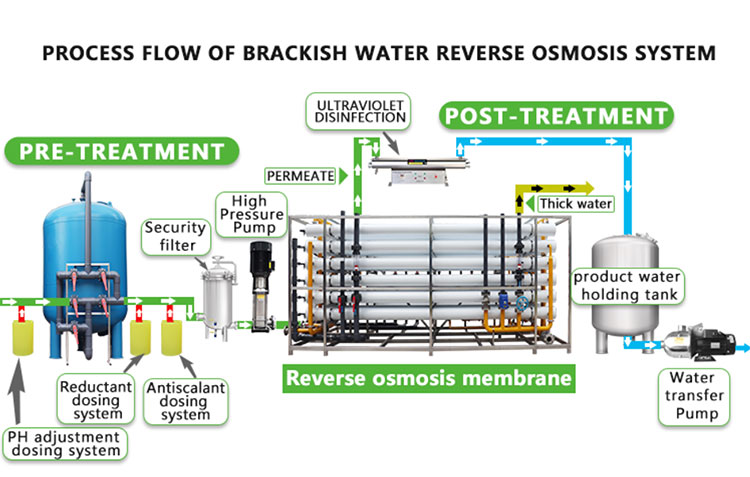
3. Reverse osmosis method
Reverse osmosis method is an efficient method to remove permanent hardness of water. Through high pressure, water passes through a semi-permeable membrane to isolate dissolved solids such as calcium and magnesium ions, bacteria, viruses, etc., thereby purifying water quality and reducing water hardness. This method is simple to operate and has obvious effects, and is widely used in drinking water treatment, industrial water and other fields.

What impact does permanent hardness of water have on life and industry?
The permanent hardness of water means that the water contains calcium and magnesium ions that are not easy to precipitate, which will bring a lot of inconvenience to life and industrial production. The impact of permanent water hardness on life and industry and the countermeasures will be discussed below.
1. Impact on life
Permanent water hardness can affect water quality and plumbing fixtures in your home. Hard water can lead to the formation of scale, which not only affects the taste and quality of drinking water, but can also damage faucets, kettles and other equipment, increasing the cost of repair and replacement.
2. Impact on industry
The permanent hardness of water can also have an impact on industrial production. In industrial production, hard water will reduce the heat transfer efficiency of heat exchange equipment, leading to increased energy consumption and higher production costs. In addition, calcium and magnesium ions in water will also react with industrial raw materials, affecting the production process and product quality.
3. Countermeasures
For the permanent hardness of water, the following countermeasures can be taken: use water softening equipment to remove hardness components in water, use appropriate processes to treat hard water, control the pH value and hardness of water, and strengthen water quality monitoring and management, etc., so as to protect life and safety. Industrial water quality and safety.